Abstract
Background: Blinatumomab is a CD19/CD3 bispecific monoclonal antibody administered as a continuous infusion and requires dose escalation in relapsed or refractory (R/R) acute lymphoblastic leukemia (ALL); whereas patients with minimal residual disease-positive (MRD) ALL are initiated at the target dose. The purpose of this study was to evaluate the real-world incidence of cytokine release syndrome (CRS) and/or neurotoxicity requiring treatment interruption between MRD and R/R ALL.
Methods: This single-center, retrospective study included patients > 18 years of age who received at least one dose of blinatumomab for MRD or R/R ALL between October 30th, 2018 to December 1st, 2021. The primary outcome was incidence of documented CRS and/or neurotoxicity requiring treatment interruption. Management of toxicities was also evaluated in patients requiring blinatumomab interruption. All results were evaluated using descriptive statistics and/or the Fisher's exact test.
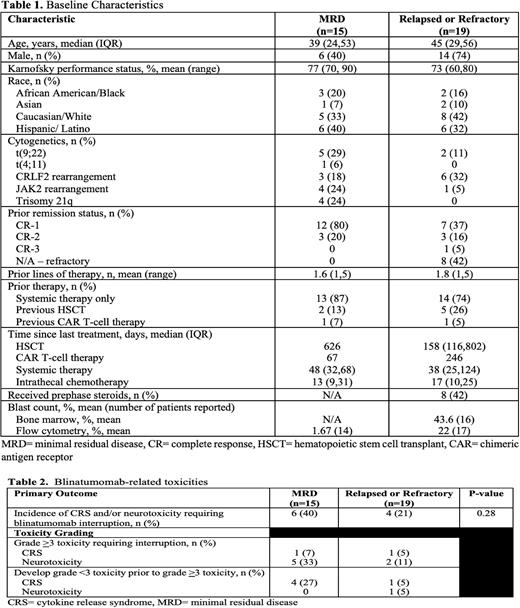
Results: Overall, 34 patient encounters met inclusion criteria. Fifteen patients were diagnosed with MRD ALL and 19 patients had R/R disease. Patient characteristics are summarized in Table 1. The median age was 39 in the MRD ALL cohort and 45 in the R/R ALL cohort with a Karnofsky performance score of at least 70 percent in both cohorts. Most patients received only one line of therapy prior to blinatumomab which was consistent between both groups as majority of patients were either refractory or in CR-1.Therapies prior to blinatumomab included hematopoietic stem cell transplant (HSTCT) and/or chimeric antigen receptor (CAR) T-cell therapy in both the ALL MRD and ALL R/R cohorts (13% and 7% vs 26% and 5% respectively).
There was no difference in the incidence of CRS and/or neurotoxicity requiring blinatumomab interruption between MRD and relapsed or refractory patients (40% vs 21%, p=0.28) (Table 2). Majority of patients requiring treatment interruption was due to Grade >3 toxicity in both groups (33% vs 11% in MRD ALL and R/R ALL respectively). Only one patient in each group required treatment interruption due to grade >3 CRS. No baseline characteristics were identified as significant risk factors for toxicities between groups; however, a history of HSCT and administration of concurrent antiepileptics did trend toward having a lower associated risk of developing severe toxicities. MRD patients were more likely to develop toxicity after a smaller cumulative dose and sooner following blinatumomab initiation (30.3 vs 183.4 hours). Time to resolution of toxicity was similar for MRD and R/R at 43 and 55 hours (p=NS). Toxicity management strategies varied among all patients with steroid treatment being the most common therapeutic management of blinatumomab-related toxicity. Exploratory outcomes suggest MRD patients were more likely to achieve remission with blinatumomab as compared to relapsed or refractory patients (87% vs 53%, p=0.035).
Conclusion: Dosing strategies for MRD and relapsed or refractory ALL did not impact the incidence of blinatumomab-related toxicities requiring treatment interruption. Neurotoxicity was the most common reason for treatment interruption. To minimize neurotoxicity risk, we are starting a clinical study to assess the efficacy of intrathecal chemotherapy as a preventative measure to help reduce toxicity and minimize treatment interruptions.
Disclosures
Solh:ADC Therapeutics: Research Funding; Partner Therapeutics: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal